You might have eaten pancakes with maple syrup this morning or sometime in the past.
You might also have enjoyed a cup of hot chocolate recently. Or perhaps, you are fond of colognes and perfumes. What is common about all these things? These are among your experiences with solutions. Most of the time, solutions are fluid but they can also be gaseous or solid.
What are solutions?
Solutions are simply mixtures of materials, one of which is a liquid or a gas. The liquid or gas, also called a fluid because it is able to flow, serves as the “solvent”. The other material in the solution is called the “solute”. The process of combining the solute and the solvent can also be called “dissolving” the solute in the solvent. The ability to dissolve is called “solubility”. The complete dissolution of one liquid in another liquid is called “miscibility”. An example of this would be vinegar and water. When the opposite occurs, the substances are called “immiscible” – an example is oil and water. So what distinguishes a simple mixture from a solution? Mixtures can have materials, which are not evenly distributed. For instance, if you try to combine coffee and sugar, you cannot actually be sure that the molecules of sugar are evenly distributed in between the molecules of coffee. In a solution, this is not so. Instead, the molecules are evenly distributed. For example, every 1 molecule of coffee would be surrounded by 3 molecules of sugar. The ratio can be 1 molecule to 1 molecule or 1:4 but the whole point is that the ratio is true for every molecule in the solution. This arrangement is called homogeneity. Therefore, a solution is also called a homogenous system. So how do you make the coffee and sugar a homogenous system? Just add water. In this example, water is the solvent while coffee and sugar are the solutes.
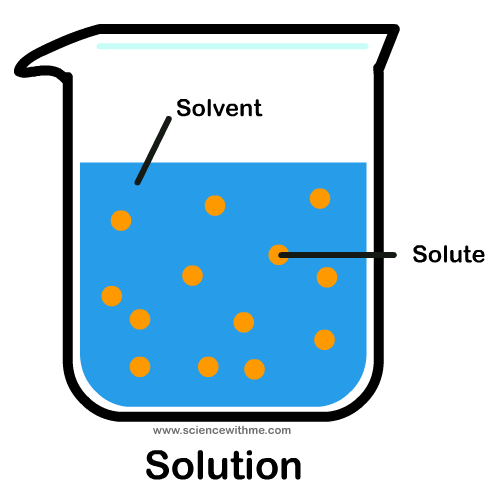
What are the properties of solutions?
There are many properties of solutions but only the most important ones will be mentioned here. A solution is homogenous, as already mentioned. When you leave it to stand, the solutes and the solvent should not separate due to gravity. A solution also has a saturation point. When you add a solute to a solvent, it can be dissolved. However, there would be a point where anything you add further would not dissolve anymore. This is called the saturation point. One way of increasing the saturation point is by increasing the temperature of the solution. Therefore, if you want more of the solute to dissolve, you can try boiling your solution. Alternatively, when you add more solute, the boiling point of the solution would increase. For instance, water boils at 100 degrees Celsius. When you add salt, this boiling point increases. Although many people do not know this, this is actually one of the reasons why it is good to add salt when cooking. Because the boiling point is increased, cooking becomes easier and faster. Additionally, the melting point of a solution would decrease when there are more solutes.
What are the kinds of solutions?
The kinds of solutions usually depend on the state of the solvent because the state of the solution is the same as the state of the solvent.
-
Gaseous Solution**.** The air we breathe in is considered a gaseous solution. The solutes are oxygen, carbon dioxide and the other gases while the solvent is nitrogen, which is present in a greater amount (75%).
-
Liquid solution. Solids, liquids and gases can be dissolved in a liquid solvent. For instance, oxygen, a gas, can be dissolved in water. In sodas, carbon dioxide is dissolved in the liquid solvent and the solution is called a carbonated beverage. Examples of liquid solutes in liquids solvents include alcoholic drinks and petroleum. In alcoholic drinks, ethanol is the solute while water is the solvent. Solid solutes such as table sugar (sucrose) and table salt (sodium chloride) can dissolve in water to form electrolytes. Electrolytes made of sodium dissolved in water or potassium dissolved in water are present in your body. When you are dehydrated, an imbalance of these electrolytes occur, which is why you need to take oral rehydration salts or you have to stay in the hospital and receive them together with a dextrose solution.
-
Solid solution. Examples of solid solutions include amalgams (mercury dissolved in gold), steel (carbon and iron), alloys (bronze) and polymers. Usually, what happens is that these materials are combined in their liquefied state then allowed to harden to form solid solutions.
Take note that some mixtures look homogenous but they are not really evenly distributed. These mixtures include colloids, emulsions and suspensions. They are not considered solutions.
